Corrosion is defined as an attack on a material as a result of chemical, frequently electrochemical reaction, with the surrounding medium. According to this definition, the term corrosion can be applied to all materials, including non-metals. But in practice, the word corrosion is mainly used in conjunction with metallic materials.
Why do metals corrode? Apart from gold, platinum and a few others, metals do not occur in the nature in their pure form. They are normally chemically bound to other substances in ores, such as sulphides, oxides, etc. Energy must be expended (e.g. in a blast furnace) to extract the metals from the sulphides, oxides, etc to obtain pure metals.
Pure metals contain more bound energy, representing a higher energy state than that found in the nature as sulphides or oxides.
As all material in the universe strives to return to its lowest energy state, pure metals also strive to revert to their lowest energy state which they had as sulphides or oxides. One of the ways in which metals can revert to a low energy level is by corrosion. The products of corrosion of metals are often sulphides or oxides.
Chemical and electrochemical corrosion
Chemical corrosion can be seen as oxidation and occurs by the action of dry gases, often at high temperatures. Electrochemical corrosion on the other hand takes place by electrode reactions, often in humid environments, i.e. wet corrosion.
All metals in dry air are covered by a very thin layer of oxide, about 100Å (10-2µm) thick. This layer is built up by chemical corrosion with the oxygen in the air. At very high temperatures, the reaction with the oxygen in the air can continue without restraint and the metal will rapidly be transformed into an oxide.
At room temperature the reaction stops when the layer is thin. These thin layers of oxide can protect the metal against continued attack, e.g. in a water solution. In actual fact, it is these layers of oxide and/or products of corrosion formed on the surface of the metal that protect the metal from continued attack to a far greater extent that the corrosion resistance of the metal itself.
These layers of oxide may be more or less durable in water, for instance. We know that plain carbon steel corrodes faster in water than stainless steel. The difference depends on the composition and the penetrability of their respectively oxide layers. The following description of the corrosion phenomenon will only deal with electrochemical corrosion, i.e. wet corrosion.
Corrosion cells
How do metals corrode in liquids? Let us illustrate this, using a corrosion phenomenon called bimetal corrosion or galvanic corrosion. The bimetal corrosion cell can e.g. consist of a steel plate and a copper plate in electrical contact with one another and immersed in an aqueous solution (electrolyte).
The electrolyte contains dissolved oxygen from the air and dissolved salt. If a lamp is connected between the steel plate and the copper plate, it will light up. This indicates that current is flowing between the metal plates. The copper will be the positive electrode and the steel will be the negative electrode.
The driving force of the current is the difference in electrical potential between the copper and the steel. The circuit must be closed and current will consequently flow in the liquid (electrolyte) from the steel plate to the copper plate. The flow of current takes place by the positively charged iron atoms (iron ions) leaving the steel plate and the steel plate corrodes.
The corroding metal surface is called the anode. Oxygen and water are consumed at the surface of the copper plate and hydroxyl ions (OH-), which are negatively charged, are formed. The negative hydroxyl ions “neutralize” the positively charged iron atoms. The iron and hydroxyl ions form ferrous hydroxide (rust).
In the corrosion cell described above, the copper metal is called the cathode. Both metal plates are referred to as electrodes and the definition of the anode and the cathode are given below.
Anode: Electrode from which positive current flows into an electrolyte.
Cathode: Electrode through which positive electric current leaves an electrolyte.
When positive iron atoms go into solution from the steel plate, electrons remain in the metal and are transported in the opposite direction, towards the positive current.
The prerequisites for the formation of a bimetal cell are:
1. Electrolyte
2. Anode
3. Cathode
4. Oxidation medium, such as dissolved oxygen (O2) or hydrogen ions (H+).
Electrode potential – Galvanic series
The electrode potential of a metal is an indication of the tendency of the metal to dissolve and corrode in a certain electrolyte.
Reference is also made to the “nobility” of the metal. The more noble the metal, the higher the potential is, the less the tendency it has to dissolve in an electrolyte.
The electrode potentials of different metals can be specified in relation to one another in galvanic series for different electrolytes.
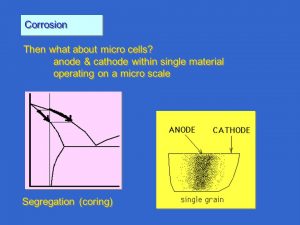
Corrosion in micro-cells
The steel-copper example has shown how corrosion takes place when two different materials are connected in an aqueous solution. How does corrosion take place on the surface of a single metal? When the surface of a metal is studied under a microscope, it will be seen that it is not a single homogeneous metal. Differences in structure and grain size occur on the surface. The chemical composition may vary and various impurities may be present.
If the electrode potential is measured across an apparently homogeneous surface, it will be found to vary considerably within areas of only fractions of a square millimetre. So cathodes and anodes, possibly small but sufficiently large to cause corrosion, can be formed on the same metal surface.
| Parameters affecting the corrosion rate Some of the most important parameters affecting the corrosion rate of metals are outlined below. Oxidizing agents: The corrosion process is conditional on an anodic reaction and a cathodic reaction taking place simultaneously. The anodic reaction causes the metal to dissolve. An oxidizing agent must be present for the cathodic reaction, and the most common agents are dissolved oxygen or hydrogen ions. If the availability of oxidizing agents is restricted, the corrosion process will be inhibited or will cease entirely. The hydrogen concentration can easily be measured as pH-value. Oxygen is normally present in water, but not in sewage due to the oxygen consuming bacteria. The electric conductivity of the electrolyte: Corrosion involves electrochemical reactions, and an increase in the electrical conductivity of the electrolyte will therefore increase the corrosion rate. In sea water the chloride content causes rapidly increased conductivity. Temperature: An increase in temperature will generally cause an increase in the corrosion rate. A rule of thumb is that temperature increases of 10°C will double the corrosion rate. Concentration: An increased concentration will normally increase the corrosion rate up to a maximum level. Higher concentration above this will not give higher corrosion rate. E.g. a chloride concentration above approximately 1500 ppm will not increase the corrosion rate. |
| Different types of corrosion Various forms of corrosion on metals and their characteristics are lined below. General corrosion General corrosion is characterized by an overall attack on the surface. The corrosion takes place without distinguished anodic and cathodic areas. The corrosion resistance of metallic materials can be illustrated in iso-corrosion diagrams. The curves indicate a corrosion rate of 0.1 mm/year in a specific liquid at different concentrations and temperatures. These diagrams are only valid for liquids in stagnant conditions. The corrosion rate will increase considerably in high velocity areas. The opposite to general corrosion is local corrosion which is divided into different types e.g. pitting, crevice and intergranular corrosion. In local corrosion, most of the metal surface is unaffected and only small areas are highly affected. It is much easier to compensate for uniform corrosion and to adopt preventive measures in the design than to make allowance for local corrosion attacks. Galvanic corrosion When two different metals are electrically connected and in contact with an electrolyte (=liquid), they will form a galvanic cell where the more noble material is cathodic and the less noble anodic. The anodic material will corrode. The electropotentials of metals can be measured in different water solutions and listed in galvanic series, as for seawater in the diagram. The corrosion rate depends on:
Pitting corrosion Typical examples of pitting corrosion can be seen on aluminium and stainless steels in liquids containing chlorides, e.g. seawater. These materials are dependent on a thin surface oxide film for their corrosion protection. Mechanical damage or an inhomogeneous spot in the oxide film could be the starting point for corrosion attacks. The conditions in the pit are characterized by oxygen deficiency and low pH, which intensifies the attack and may also render it self-sustaining. The rate of pitting corrosion can be very high with the attack being localized to a considerable depth. Pitting corrosion is most likely to occur in stagnant water. Stainless steels as AISI 316L (M 0344.2343.02) and AISI 329 (M 0344.2324.02) are not resistant to pitting corrosion in seawater. Other higher alloyed stainless steels such as UNS S31254 are considered to be resistant in seawater. Crevice corrosion The mechanism for crevice corrosion is similar to that for pitting corrosion. Crevice corrosion takes place in confined liquid filled slots and crevices where the liquid circulation is prevented. Once corrosion has appeared, conditions in the crevice are changed; e.g. the pH-value is reduced and the chloride concentration increase. Accordingly the corrosiveness of the confined liquid will increase. Crevice corrosion mainly appears on stainless steel and aluminum in liquids containing chlorides. Intergranular corrosion Intergranular corrosion occurs between the grain boundaries inside a metal. This type of corrosion is well known for stainless steels which have been soaked for an excessive period of time at temperatures between 500 and 800 °C. At this temperature chromium will react with carbon at the grain boundaries and form carbides. This causes chromium depletion in the immediate vicinity of the grain boundaries. If the chromium content falls below 12 %, corrosion can easily start. Stress corrosion corrosion is a combined effect of tensile stresses, either internal or applied, and a local corrosion attack. Tensile stresses arise for example during cold work of steel sheet or as a result of directly applied load. Stress corrosion is generally connected with austenitic stainless steels in contact with liquids containing chlorides. Cracks are however unlikely to occur below +60° C. Carbon and low alloy steels may be subject to stress cracking in caustic soda solutions at high concentrations and temperatures. To avoid stress corrosion, tensile stresses should be removed, e.g by heat treatment after cold working or welding. Stress corrosion can also be avoided by the choice of a resistant material.Erosion corrosion Erosion corrosion is a combination of electrochemical corrosion (i.e. general corrosion) and the action of a high speed fluid, eroding the corrosion product. The pits formed by erosion corrosion usually have bright surfaces free from corroded material. The attacks are generally localized to areas with turbulent flow and are promoted by gas bubbles and solid particles. Cavitation corrosion Cavitation corrosion appears in areas where vapour bubbles are formed due to low pressure. When the bubbles implode on a surface the protective oxide is destroyed and eroded away and after that built up again. The process is repeated and characteristic deep holes of cavitation corrosion are formed on the surface. It can usually be seen on the trailing edge of impellers and propellers. Selective corrosion Selective corrosion occurs in metals in which the alloying elements are not uniformly distributed. Typical examples of this type of corrosion are:
|
CGPCS Notes brings Prelims and Mains programs for CGPCS Prelims and CGPCS Mains Exam preparation. Various Programs initiated by CGPCS Notes are as follows:-