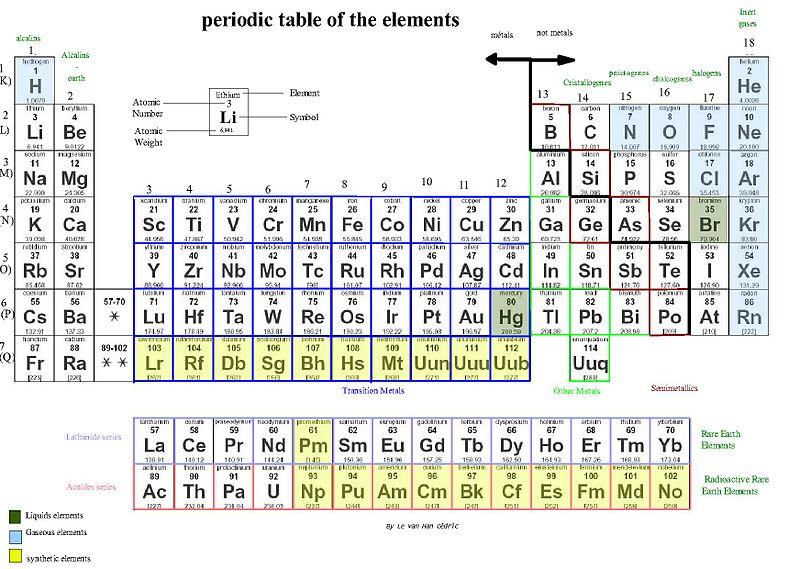
Metal
In chemistry, a metal is an element that readily forms positive ions (cations) and has metallic bonds. Metals are sometimes described as a lattice of positive ions surrounded by a cloud of delocalized electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. On the periodic table, a diagonal line drawn from boron (B) to polonium (Po)
separates the metals from the nonmetals. Most elements on this line are metalloids, sometimes called semi-metals; elements to the lower left are metals; elements to the upper right are nonmetals. A modern definition of metals is that they have overlapping conduction bands and valence bands in their electronic structure. This definition opens up the category for metallic polymers and other organic metals, which have been made by researchers and employed in high-tech devices. These synthetic materials often have the characteristic silvery-grey reflectiveness of elemental metals. The traditional definition focuses on the bulk properties of metals. They tend to be lustrous, ductile, malleable, and good conductors of electricity, while nonmetals are generally brittle (for solid nonmetals), lack lustre, and are insulators.In
the periodic table, you can see a stair-stepped line starting at Boron (B), atomic number 5, and going all the way down to Polonium (Po), atomic number 84. Except for Germanium (Ge) and Antimony (Sb), all the elements to the left of that line can be classified as metals. These metals have properties that you normally associate with the metals you encounter in everyday life:- They are solid (with the exception of mercury, Hg, a liquid).
- They are shiny, good conductors of electricity and heat.
- They are ductile(they can be drawn into thin wires).
- They are malleable(they can be easily hammered into very thin sheets).
All these metals tend to lose electrons easily. The following figure shows the metals.
CGPCS Notes brings Prelims and Mains programs for CGPCS Prelims and CGPCS Mains Exam preparation. Various Programs initiated by CGPCS Notes are as follows:-