Primary and secondary cells their properties and drawback
Primary Cell
Primary cells are those that cannot be recharged and needs to be discarded after the expiration of their lifetime. If the electrolyte is not in liquid form, we are talking about dry cells.
Primary cells usually have high energy density, capacity, are slowly to discharge, easy to use and not excessively expensive. Alkaline are probably most commonly used primary batteries. They usually have zinc anode, carbon cathode and electrolyte. The voltage curve for discharging alkaline batteries is very steep (almost linear.
When the battery empties, its voltage drops almost linearly. Such cells are therefore not suitable for digital cameras, as they require relatively high voltage for their operation. Alkaline battery is therefore showed as “empty” after a few hours of using it,
although in reality it isn’t. Read more: Difference Between Primary Cell and Secondary Cell | Difference.Most of the primary cells are comfortable, always available and environmentally friendly. They also have extremely high energy density.
Only in recent years, the rechargeable cells have reached the density of primary cells, but conventional alkaline batteries produce almost 50% more energy than a comparable Li-Ion secondary cell.
These cells continuously charge
and supply all kinds of devices, from basic, all known devices to specialized devices and applications. Primary cells are most commonly used in wristwatches, remote controls, children’s toys and non-demanding entertainment electronics. They are also used wherever the charging is impractical or impossible, in cases of military and rescue techniques, in difficult to access control stations, and the like.Because of low prices, they are especially suitable where the power requirements are not very high, where the devices do not require a high level of energy for their operation, and need just a constant voltage.
Primary cell
Secondary Cell
With the rise of portable devices such as laptops, smartphones or MP3 players, there is a growing demand for good batteries that we will not have to change every couple of days. And here we come to the need of rechargeable (secondary) cells.
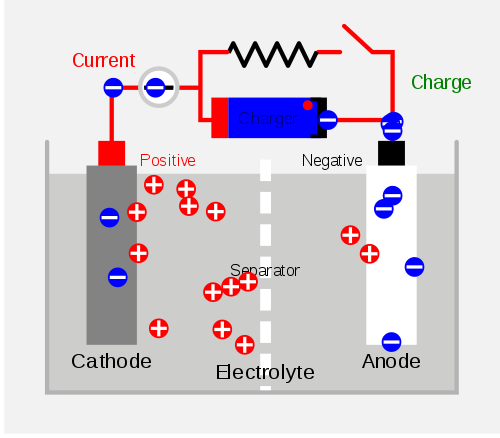
The principle of their work is actually the same – electricity is generated through a chemical reaction involving anode, cathodes and electrolytes, but the difference is in the chemical composition of the cells contained in the batteries.
Here we have the case that the chemical reaction is reversible. When the battery “consumes” (or when the negatively charged ions go to the positive side of the battery), the battery is charged. By connecting a secondary cell to an external source of electrical power (e.g., electricity), the opposite process occurs – the negatively charged ions return to the negative side of the battery and can be used again.
The most commonly used secondary batteries on the market are: lithium-ion (LiOn), nickel-metal hydride (NiMH), and nickel-cadmium (NiCd). When talking about secondary batteries, we must say that they are not all equal. NiCd (nickel cadmium) were the first secondary batteries that were used everywhere in the world, but they had one small problem – “memory effect”.
The memory effect means that you have to refill and empty them every time, otherwise they will lose their capacity quickly. This has led to a situation where people are switching to Nickel–metal hydride (NiMH). They had somewhat greater capacity and did not “suffer” from the memory effect, but their lifespan was short – you could fill them up and empty them around 100 times.
And finally, the most popular LiOn batteries are used today, which have proved to be the best variant. Perhaps their capacity is somewhat smaller, but the technology of making them is simpler than the ones previously mentioned, they are smaller, easier and have a cycle of 1000 charging and discharging.
Leclanche cell
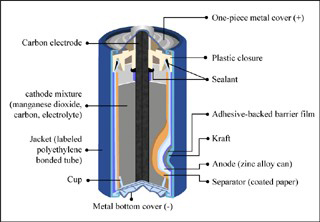
Leclanche cell is a primary cell, handy for sporadic use, with positive anode of zinc encompassed by a mixture of manganese dioxide and powdered carbon in a pot, which is porous. The pot and the negative zinc terminal remained in a container holding ammonium chloride solution. The electromotive force (emf) is nearly 1 -4 volt.
Going back to its history, the Leclanche cell was invented by the French engineer Georges Leclanche in 1866. Leclanche’s battery, additionally called a zinc-carbon battery, made use of an alternate type of cell than its antecedents. Rather than lead, the French engineer utilized zinc and a carbon-manganese dioxide mixture for his terminals. He additionally made use of ammonium chloride instead of the sulfuric acid that had been used as the preferred electrolyte. The changes he made to his battery made the cell less dangerous and lighter than the most commonly used Plante model.
- Types of Leclanche’s cell include: zinc (Carbon cathode)
- zinc chloride (Ammonium chloride electrolyte reinstated by zinc chloride)
- alkaline manganese (Ammonium chloride terminal displaced by potassium hydroxide)
The process which generates power in a Leclanché cell starts when zinc particles on the surface of the anode oxidize, i.e. when zinc atoms surrender their valence electrons to end up becoming the positively charged particles. The zinc particles move far from the anode while leaving their electrons on its surface that makes the anode more negatively charged than the cathode. At the point when the cell is associated in an outer electrical circuit, the excess electrons on the zinc anode gush through the circuit to the cathode made up of carbon. This flow of electrons frames the electric current.
After going through the entire circuit, when electrons enter the carbon rod, which is the cathode, they join together with water and MNO2 (Manganese Dioxide) that further reacts with each other to produce negatively charged hydroxide ions and manganese oxide(Mn2O3). Whole of this process is accompanied by secondary reaction, wherein the negative hydroxide ions react with positive ammonium ions in the electrolyte of ammonium chloride to produce molecules of water and ammonia.
CGPCS Notes brings Prelims and Mains programs for CGPCS Prelims and CGPCS Mains Exam preparation. Various Programs initiated by CGPCS Notes are as follows:-