Endothermic Reactions
Endothermic reactions are those chemical reactions where energy is absorbed by the system from the surroundings mostly in the form of heat. The concept is applied in the physical sciences like chemical reactions where hear is converted to chemical bond energy by way of experiments. Common examples of endothermic reactions are cooking an egg, photosynthesis, and evaporation. This reactions process accounts for the enthalpy change of a reaction only. The overall energy analysis of any reaction is the Gibbs free energy that includes temperature and entropy in addition to the enthalpy. The point to mention here is that endothermic reactions release energy always in the form of heat only. Moreover, the products have more energy as compared to the reactants. The end result of any endothermic reaction is in an increase in chemical potential energy. The endothermic reaction always needs a greater amount of energy to break the existing bonds in the reactants in order to need the new bonds form in the products. In a nutshell in the endothermic reactions process, less energy is added to the environment as compared to the amount of energy absorbed to initiate and maintain the reaction.
Exothermic Reactions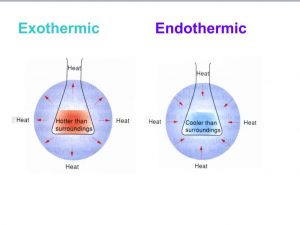
An exothermic reaction is a chemical reaction that releases energy in the form of heat, light, sound or even electricity. It can be expressed as the reaction where reactants results in products and energy. Overall it adds energy to the surroundings. Moreover, it is the energy that is needed to start the reaction process and is always less than the energy released. It ‘s hard to measure the amount of energy released during the chemical process. However, the enthalpy change of a chemical reaction is easier to work, and it always equals the change in internal energy of the system and the amount of work required to change the volume of the system against constant ambient pressure. The concept of exothermic reactions process is applied in the physical sciences to chemical reactions where the energy of chemical bond is converted into thermal energy. It explains two kinds of chemical systems or reactions found in nature. In a nutshell in the overall process, more energy is added to the environment as compared to the amount of energy that was absorbed to initiate and maintain the reaction.
Key Differences
- Endothermic reactions absorbed the heat while exothermic reactions give out the heat.
- In the case of endothermic reactions, the content of energy of the reactants is always less than the products while it happens reverse in the case of the exothermic reactions.
- The change of enthalpy for endothermic reactions is always positive while it tends to negative in case of AH in a change of enthalpy in the exothermic reactions.
- In endothermic reactions, small positive free energy while in exothermic reactions large negative free energy.
- All endergonic reactions are exothermic while all exergonic reactions are exothermic.
- The common examples of endothermic reactions are cooking an egg, photosynthesis, and evaporation. The common examples of exothermic reactions are a fireplace, respiration, and combustion.
- Endothermic results in an increase in chemical potential energy while exothermic reactions result in a decrease in chemical potential energy.
- Exothermic reactions are hotter than surroundings while endothermic reactions are cooler than surroundings.
- In endothermic reactions, energy is always present in the form of heat while in the case of exothermic reactions; energy is always present in the form of heat, electricity, sound or light.
- In the endothermic reactions process, less energy is added to the environment as compared to the amount of energy absorbed to initiate and maintain the reaction. In exothermic reactions process, more energy is added to the environment as compared to the amount of energy that was absorbed to initiate and maintain the reaction.
CGPCS Notes brings Prelims and Mains programs for CGPCS Prelims and CGPCS Mains Exam preparation. Various Programs initiated by CGPCS Notes are as follows:-